Crystals from Wart Remover and Nail Polish?
I have been experimenting with combinations of Salicylic Acid (a common ingredient in wart & corn remover), Clear Nail Polish, Acetone and Methylated Ethanol. By slightly changing the ratios of these ingredients, and even changing how the solutions are applied to slides, the results can change dramatically. Here are results as I played with amount of nail polish in the recipe. Images are focus stacked from short videos traveling through focus, and were taken with cross-polarized light using a 10/0.25 Plan Phase Contrast Objective (FOV: 1.4 x 0.8mm). A scrap of clear cellophane was inserted into the light path as a retarder to impact and enhance the resulting colors.
Base Recipe
- Acetone – 6g (about 0.5ml)
- Salicylic Acid Powder – 0.13g
2 drops of nail polish (0.05g)

4 drops of nail polish (0.10g)
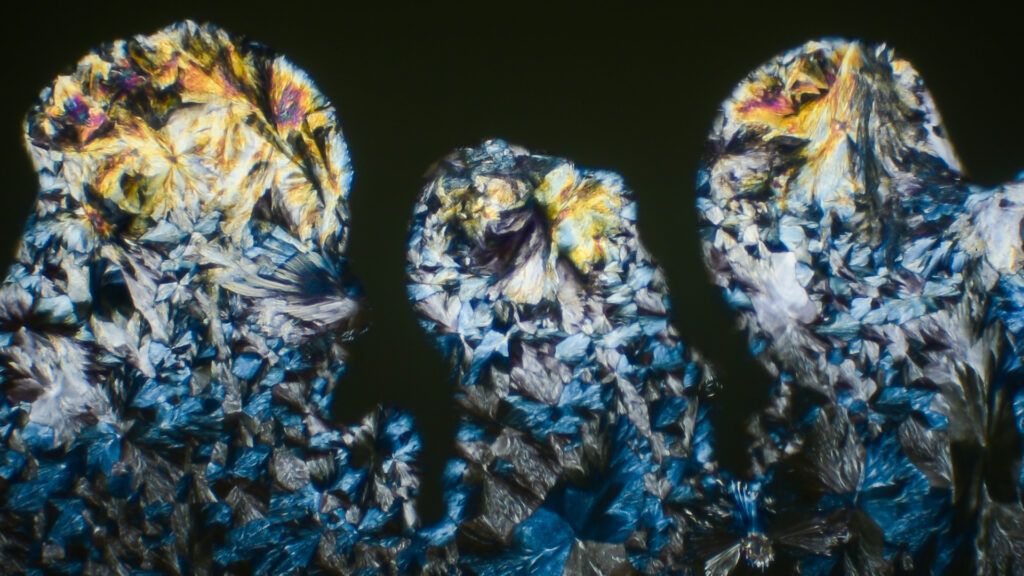
6 drops of nail polish (0.15g)

8 drops of nail polish (0.20g)

10 drops of nail polish (0.25g)


Additional experiments were done making slight modifications to the recipe and procedure:
- Acetone – 7g
- Salicylic Acid Powder – 0.225g
- 7 drops of nail polish (0.175g)
This time, slides were warmed for 1 minute on an electric coffee cup warmer, then drops of the solution were splashed onto the slide from a height of about 2 feet. This method provided thinner areas of crystallization.



One final experiment (for which I did not measure the ingredients) included a 2:1 ratio of Acetone:Methylated Ethanol, with the other ingredients approximately the same as in other recipes (I used 5 drops of Nail Polish). The results were quite different from the curled crystals that didn’t include Methylated Ethanol, but took on an amazing array of colors.

Notes on preparation:
- Solutions were warmed for about 7 seconds at full power in a microwave and then stirred to fully dissolve all ingredients
- Solutions were mixed in a small beaker
- Solutions were added to slides using a new micropipette tip each time so avoid contaminating each new combination
- Attempts to include a solution of dissolved Lactic Acid proved futile. Apparently the solution I purchased suspended the Lactic Acid into something with an oily consistency that prevented evaporation and crystallization on a slide, even when heated or when allowed to sit for several days.


